“The art is at one’s surface and symbol; those who go beneath the surface do so at their own peril”
~ Oscar Wilde
Introduction
The surface is seen, the surface is perceived, and the surface defines the aesthetic impression of the object.
The aesthetic experience of a fenestration object is an interlinked and inseparable union of the sensory and contemplative responses. Illuminating the modern surface condition, façades are becoming virtual screens reinventing the art of projection.
While the surface of a façade is defined by glass and framing material together with cladding if any, this article’s prime focus is to delve into the various possibilities of surface finishes available for aluminium and uPVC – so far the most popular choice of materials for window and façade frames.
Definition:
Surface finishing is the process of enhancing the properties of aluminium in all its forms to give it a decorative visual appearance, improve the metal’s excellent corrosion resistance and enhance its durability.
SELECTION CRITERIA FOR SURFACE FINISH ON ALUMINIUM
Selection of a particular surface finish is usually focused on the appearance requirements, quality performance, cost and compliance. There are several coating options that are available for the architectural aluminium market and a number of factors govern their specification in a particular project. For instance –
- Appearance – solid, metallic, bright, gloss, matt, brushed, etc.
- Application – interior or exterior
- Alloy and temper of aluminium to be used: Various combinations of constituent elements cause each aluminium alloy to react differently in the coating process. As a result, each alloy or alloy series yields a different appearance, even if subjected to an identical coating process.

IMPORTANCE OF SURFACE FINISH
The surface finish of aluminium has a great influence on the visual character of the window/façade and should be considered carefully during the design process. Regarded for too long simply as a means of corrosion protection, surface treatments in fact modify the condition or properties of the surface of the metal in a variety of ways:
- They modify certain properties of the metal surface such as surface hardness and abrasion resistance
- Alter its appearance:
- Contribute to the decoration of the metal cladding
- Provide further protection from corrosion while imparting definite durability.
Colours and finishes are critical components of an architect’s design. Decades after construction the finishes of building components should ideally be just as pure as when the projects were completed. Colours and gloss levels should be minimally altered and surfaces unblemished despite constant exposure to the sun, wind and airborne contaminants.
CORROSION IN ALUMINIUM
One of the most important reasons why aluminium needs to be coated or treated is to increase its natural corrosion resistance and enhance its durability.
Although aluminium enjoys the advantage of a hard, inert oxide film (fig. 3) that is formed instantaneously when the metal is cut and abraded which inhibits corrosion, depending on various physical conditions this natural oxide layer may be insufficient protection:
- Corrosion depends on the time of exposure to moisture and relative humidity and temperature.
- Aluminium is more prone to corrode in coastal areas. The corrosion is inversely proportional to the distance from the sea
- For aluminium aggravating conditions can be a strong sulphurous environment, the occurrence of chloride salts and stagnant water.
- Pitting corrosion on aluminium is unsightly but it is not structurally significant
fig. 4 Corrosion in aluminium
SURFACE TREATMENT ON ALUMINIUM
Colours and finishes are critical components of an architect’s design. Decades after construction the finishes of building components should ideally be just as pure as when the projects were completed. Colours and gloss levels should be minimally altered and surfaces unblemished despite constant exposure to the sun, wind and airborne contaminants.
A quality finish is essential to achieve longevity of the façade.
Broadly speaking there are three main types of surface treatments popularly used on aluminium –
- Anodizing
- Powder Coating
- PVDF (Poly Vinylidenedi-Fluoride)Coating
Besides these, there are available options for liquid paint and lamination as well.
Pretreatment:
Regardless of the finish chosen for a project, the critical first step in the application process is pre-treatment to prepare the surface for coating. (fig. 6)
Objectives of Pre-treatment:
- Removal of impurities including dust, welding splatter, scale, grease and oil
- Conditioning of the surface for optimum adhesion of the coating film
- Obtaining uniformity through the entire treated surface
The pre-treatment for surface preparation includes the following two main steps:
1. Cleaning – Mechanical or chemical: aluminium surface has a natural tendency to undergo natural oxidation. This layer has to be removed before powder coating. Apart from this, the metal surface must also get rid of oil, dirt and grease in order to achieve perfect bonding of powder coating.
Mechanical cleaning includes methods like scratching, brushing and sandblasting. This not only removes surface impurities but also eliminates scratches and surface irregularities. Chemical cleaning includes removal of dirt, oil and grease by means of applying specific chemicals
- Alkaline cleaning – Mild alkaline cleaning agents can be used for cleaning and etching the surface of aluminium. However, if there is an anodizing layer on aluminium, caustic soda-based strong alkaline cleaner has to be used. This leaves a black smut on the surface which needs to be removed by dipping the extrusion into a weak Nitric acid bath. This process is called de-smutting.
- Acidic Cleaning –The most commonly used substance for acid cleaning of aluminium is phosphoric acid. The acid helps in removing the oxide layer without the formation of residual black smut. Therefore an additional step of de-smutting can be avoided.
2. Conversion Coating – cleaned aluminium is highly reactive and very prone to oxidation and or corrosion. In order to prevent the formation of an oxide layer once again which had been removed earlier by the process of cleaning, conversion coating has to be done.
Conversion coating methods prior to powder coating are divided into 2 types, phosphating and chromating. Each type of pre-treatment is then subdivided into 2 two types.
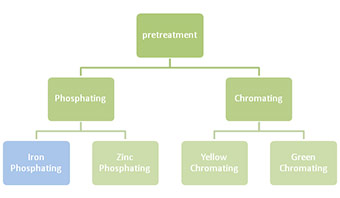
- Phosphating: Phosphating is an application of an iron or zinc phosphate coating onto the aluminium surface. This significantly increases the performance of the finished coating. The aluminium is subjected to an acidic bath and a chemical conversion forms a complete film on the surface, changing its chemical and physical nature.
- Iron Phosphating: Iron phosphate is the thinnest phosphate film. During the phosphating process, a flat or amorphous metal phosphate topcoat will be formed on the firstly developed iron oxide base. Once treated, the metal surface will have a blue iridescent or blue-gold iridescent colour, depending on the coating weight and the base metal.
- Zinc Phosphating: Zinc phosphate is a non-metallic, crystalline coating that chemically adheres to the surface of aluminium. Zinc phosphate forms a uniform coating which increases the adhesion properties and gives better coating in recessed areas along with better corrosion resistance. Unlike iron phosphate which comes from the surface of aluminium substrate, zinc phosphate comes solely from the mixture solution itself
- Chromating: It is the most used conversion coating process for aluminium profiles which are going to be powder coated. The chromate layer enhances the adhesion between the metal surface and the lacquered product. If the chromate layer is thick, it will give a very good corrosion resistance, but the adhesion will be poorer compared to a thinner layer. However, the chromate layer which is too thin will result in good adhesion, but poorer corrosion resistance. Chromatin is an elaborate process that includes the following steps:
- Degreasing
- Rinsing
- Etching
- Rinsing
- Deoxidisation
- rinsing (double rinsing)
- chromatin
- rinsing
- drying
- powder coating
- curing
Green Chromating:
The phosphochromating or green chromating process is carried out in phosphochromate baths. The main components of the bath are phosphoric acid and/or an acid phosphate, hydrofluoric and chromic acid or another source of hexavalent chromium.
The bulk of the phosphor chromic coating consists of a hydrated from of chromium phosphate with smaller amounts of chromium oxide. As the aluminium oxides and fluorides are present in the intermediate surface regions, the chromium oxides are concentrated towards the aluminium surface.
The phosphochromate conversion coatings are green in colour. As the coating weight increases from 0.4 to 1.2 g/m2, the intensity of the colour changes from iridescent green to dark green. The coating should be as even as possible, adhere to the substrate and free from powder. Nevertheless, the colour and its uniformity may differ in visual examination, depending on the material and its surface conditions.
Yellow Chromating:
This chromating process can be done in either non-accelerated or accelerated chromate baths. The former is essentially composed of chromium (III) oxides (another source of chromium ions) and of hydrogen fluorides (complex hydrofluoric acids, or their salts). To obtain the desired pH of about 1.8 to 2.1, nitric acid is usually added. Other substances are present in appropriate formulations to achieve better conditions in industrial use. The temperature of the bath should be around 25oC. In the accelerated baths, potassium ferricyanide is usually added, but many other variations have been developed. Cyanides are less used due to environmental concerns.
From the examination of the chromate layer, it is known that a thin outer layer consisting of chromium ferricyanide and hydrated chromium oxide conceals the bulk of hydrated chromium oxide coating. Small amounts of aluminium oxide and fluoride are present in the interface between the film and the aluminium. A yellow layer is obtained from this chromate conversion coating, with a variation of intensity from iridescent yellow to golden tan as the coating weight increases from 0.4 to 1.0 g/m2. The limit value of 1.0 g/m2 has been established because of the powdering phenomenon of the conversion coating if exceeded.
Increased environmental awareness has led to the development of certain chrome-free pre-treatments as well.
ANODIZING
Combining science with nature, anodizing is an electrochemical process that thickens and toughens the naturally occurring protective oxide layer in aluminium. The resulting finish, depending on the process, is the second hardest substance known to man, second only to the diamond. The anodic coating is part of the metal but has a porous structure (Fig. 9) that allows secondary infusions (organic and inorganic colouring, etc.) The performance of the anodizing process is well-known amongst architects and specifiers and is used on many of the best-known aluminium structures in the world.
Process:
Anodising is an electrolytic process that produces a dense, chemically stable protective aluminium oxide film that is an integral part of the underlying aluminium. This greatly increases the corrosion resistance and its exceptional hardness protects any underlying surface finish produced mechanically or chemically or by polishing, etching or brightening. The anodising itself can be achieved through several processes:
- Barrier Anodising
- Sulphuric Acid Anodising
- Chromic Acid Anodising
- Hard Anodising
- Phosphoric acid anodising
The sulphuric acid process is most often used for architectural purposes because the film produced gives outstanding corrosion and abrasion resistance, and being transparent, can be coloured without adversely affecting its excellent properties.
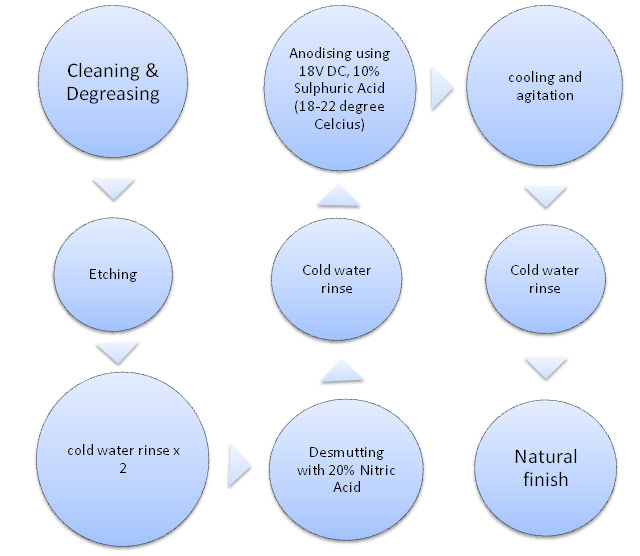
Steps of a Typical Anodizing Process(chart 1):
Cleaning and Etching:
Preceding the anodising process, wrought alloys are cleaned in either a hot soak cleaner or in a solvent bath and may be etched in sodium hydroxide (normally with added sodium gluconate), ammonium bi-fluoride or brightened in a mix of acids.
Anodizing:
The anodised aluminium layer is grown by passing a direct current through an electrolytic solution, with the aluminium object serving as the anode (the positive electrode). The current releases hydrogen at the cathode (the negative electrode) and oxygen at the surface of the aluminium anode, creating a build-up of aluminium oxide. The voltage required by various solutions mostly falls in the range of 15 to 21 V. Higher voltages are typically required for thicker coatings formed in sulphuric and organic acids. The anodising current varies with the area of aluminium being anodised and typically ranges from 0.3 to 3 amperes of current per square decimetre (20 to 200 mA/in)
The acid solution slowly dissolves the aluminium oxide. The acid action is balanced with the oxidation rate to form a coating with nano-pores, 10-150 nm in diameter. These pores are what allow the electrolyte solution and current to reach the aluminium substrate and continue growing the coating to greater thickness beyond what is produced by auto-passivation. However, these same pores will later permit air or water to reach the substrate and initiate corrosion if not sealed. They are often filled with coloured dyes and/or corrosion inhibitors before sealing. Because the dye is only superficial, the underlying oxide may continue to provide corrosion protection even if minor wear and scratches may break through the dyed layer.
The detailed process is described in chart 2.
(I have used smart art in Word. If Ramesh can use a better tool to show the flow chart, it will be good)
Colouring:
Once the desired anodised film is formed, the colouring is done by using either of 2 methods:
- Electrolytic Colouring
- Organic or Inorganic Dyeing
Sealing:
In all the anodizing processes, the proper sealing of the porous oxide coating is absolutely essential to the satisfactory performance of the coating. The pores must be rendered non-absorbent to provide maximum resistance to corrosion and stains. This is accomplished through a hydrothermal treatment in proprietary chemical baths or by capping the pores via the precipitation of metal salts in the pore openings.
Characteristic properties of an anodising film:
- Resistance to corrosion against a variety of atmospheres and environments.
- Offers a variety of colours for external and internal applications.
- Enhancement of optical properties such as reflectivity and brightness.
- Specular reflectivity (pearl finish) of +85% can be achieved.
- Bright aluminium reflects over 80% of light and about 90% of heat radiation that falls on it.
Advantages of Anodizing:
As an integral part of the material, the finished aluminium will not chip, crack, peel or blister
- Sustainable building construction for more than 80 years
- Excellent weather and abrasion resistance
- Resistant to salt air environments
- Metallic lustre finish
- Minimal coating erosion over time
- Remarkable substrate adhesion
- Water-based process, generates no harmful waste or VOC
- Incredibly long life cycle
- The anodizing process uses a series of dip tanks enabling efficient material utilization
Limitations of Anodizing:
- Limited range of colour and Gloss as compared to spray paints
- Handling and disposal is a challenge considering several acids and other hazardous products that are used in the process
- Requires high-level process control in order to achieve consistent colour across large surfaces
- The anodized surface can be stained by cement
- If not sealed properly, pitting corrosion is a frequent occurrence
POWDER COATING
Powder coating is an advanced method of applying a decorative and protective finish to aluminium. The process result is a uniform, high quality and attractive finish possible in a variety of colours. Powder-coated surfaces are more resistant to chipping, scratching, fading and wearing than other finishes. Colour selection is virtually unlimited with high and low gloss, metallic and clear finishes available. And colours stay bright and vibrant. Texture selections range from smooth surfaces to a wrinkled or matt finish and rough textures designed for hiding surface imperfections.
Long recognized for their excellent finish quality in appearance and performance, reasonable cost per applied square foot and relative ease of application, organic powder coatings on aluminium extrusion for the architectural market have quickly gained popularity. Some early attempts to put powder into harsh outdoor environments were not very successful. However, development in powder technology has advanced considerably in the last few years. In recent times, through resin development, it has become possible to produce powder coatings with exterior durability that can meet the AAMA 2604 specification (i.e. five years of Florida exposure), making it an excellent choice for applications for extremely hostile conditions.
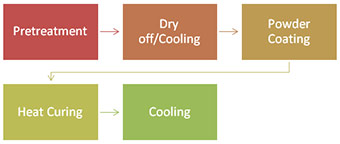
Powder Coating Process(chart 3):
The pre-treated aluminium components are racked to a conveyor, which is earthed. A powder spray gun (Fig. 11) is set to produce a cloud of powder particles with a strong electrostatic charge. The charged particles are attracted through the electrostatic field to the face of the product and to the sides and back along the lines of force in the electrostatic field. After the powder has been applied, the coating is stoved at temperatures specified by the powder manufacturer.
Polyester powder coatings are available in a vast range of colours. These may be specified from the international RAL colour chart, or the individual colour ranges of powder manufacturers and coaters. Specifiers also specify the gloss level required, matt surface being preferable for most architectural finishes.
Characteristic Properties of Powder Coatings:
- Tough & durable
- Excellent corrosion resistance
- Impact resistance
- Attractive and colourful with an excellent finish
- Solvent-free and environment-friendly
Advantages of Powder Coatings:
- No VOC emission, so virtually pollution-free
- Unused or over-sprayed powder can be recovered
- Minimal wastage and easy and safe disposal
- Superior cured film properties
Limitations of Powder Coatings:
- Despite having excellent flow properties, the powder cannot fully even out the surface imperfections
- To apply the powder, the items have to be hanged via one or more jigging points.
- Faraday’s Cage Phenomenon: Powder initially adheres during coating by electrostatic forces, but this phenomenon can counter the action on components with narrow recesses, slots or sharp, enclosed corners.
Suggested Read: PVDF coating vs. powder Coating
PVDF COATING
These are solvent-based paints with a low solids level. This type of coating is created by polymerizing vinylidene difluoride. It’s a speciality plastic that belongs to the family of fluoropolymers and is used in applications that require high levels of purity and strength, as well as those that require excellent resistance to acids, solvents, bases, and heat. PVDF has a fairly low melting point, which can be beneficial as well in certain applications,
PVDF comes in two grades:
- 70% PVDF and 30% other resins, acrylic usually predominating.
- 50% PVDF and 50% resin
50% grade does not meet AAMA 2605-98 (weathering requirement of 10 years Florida) in all colours. This grade has a durability that is less than the 70 per cent types but is still comparable to super durable powder coatings, meeting the AAMA 2604-98 weathering requirement of five years Florida Chemical resistance and resistance to UV light are the strengths of PVDF coatings. PVDF has a definitive edge in the curtain wall and metal roofing markets because of its weather ability, and because of the wide variety of colours available.
Advantages of PVDF Coating:
- PVDF is relatively chemically inert and will outlast anodizing in corrosive environments. Window washers can be less discriminating about the types of chemicals they use to clean a building. If extremely corrosive cleaners are used, however, even PVDF will show signs of damage.
- PVDF coatings offer nearly an unlimited selection of colours and are easy to manufacture in small batches.
- Colour consistency is usually better than with anodizing.. Metallic metal flake coatings are especially prone to colour variation. To improve colour consistency, all the metal for a project should be painted in one set-up.
- PVDF has exceptional resistance to fading from sunlight. In addition, they can produce high “metallic” appearances
Limitations of PVDF Coatings:
- In spite of their excellent weathering characteristics, PVDF coatings tend to scratch and marble if abraded or scuffed. Fabricators and installers report significant levels of “on-site” damage that require touching up and repair.
- PVDF coatings are expensive both in material and application costs. The key difference in terms of application costs is that PVDF is applied as a multi-coat system. The minimum is a two-coat finish, but such PVDF coatings can only be produced as a matt finish. If higher gloss levels are required, one or more additional layers of clear topcoat must be applied. This increases the cost substantially.
CONCLUSION
There are many options available for finishing aluminum which is why it is such a popular construction material. The question of which finish to apply is not always an easy decision because of all the options available. The readers can draw conclusions based on their preferences. With newfound awareness about quality standards like those from Qualicoat and GSB, the surface finish industry is set to scale new heights and greater paradigms.
The subject of surface finishes is vast. Although in this edition we have tried to cover the most popular finishes for aluminium, the next issue will see a detailed description of the surface finish choices possible and available for uPVC.
References:
- White paper on pretreatment
Oxygen - Powder coating on aluminium extrusion
Robert Talbert - Choosing sustainable finishes
AIA CES System - How to decide between Anodizing, PVDF & Powder
Southern Aluminium Finishing Co. - Aluminium Handbook 2, Section 3
AluminiumVerlag - Aluminium Extrusions Council
- American anodised council
- Aluminium surface finishing user guide
AFSA - Importance of Quality Finishing for Longevity of Façade
Australian Aluminium Finishing














